

* Consistent results across a broad range of HR+/HER2– eligible patients with statistically significant improvements in iDFS (EBC), PFS and OS (ABC) vs AI in the NATALEE study and ET in the MONALEESA studies.1 KISQALI is not recommended to be used in combination with tamoxifen.1
See why physicians are choosing KISQALI + ET for their patients
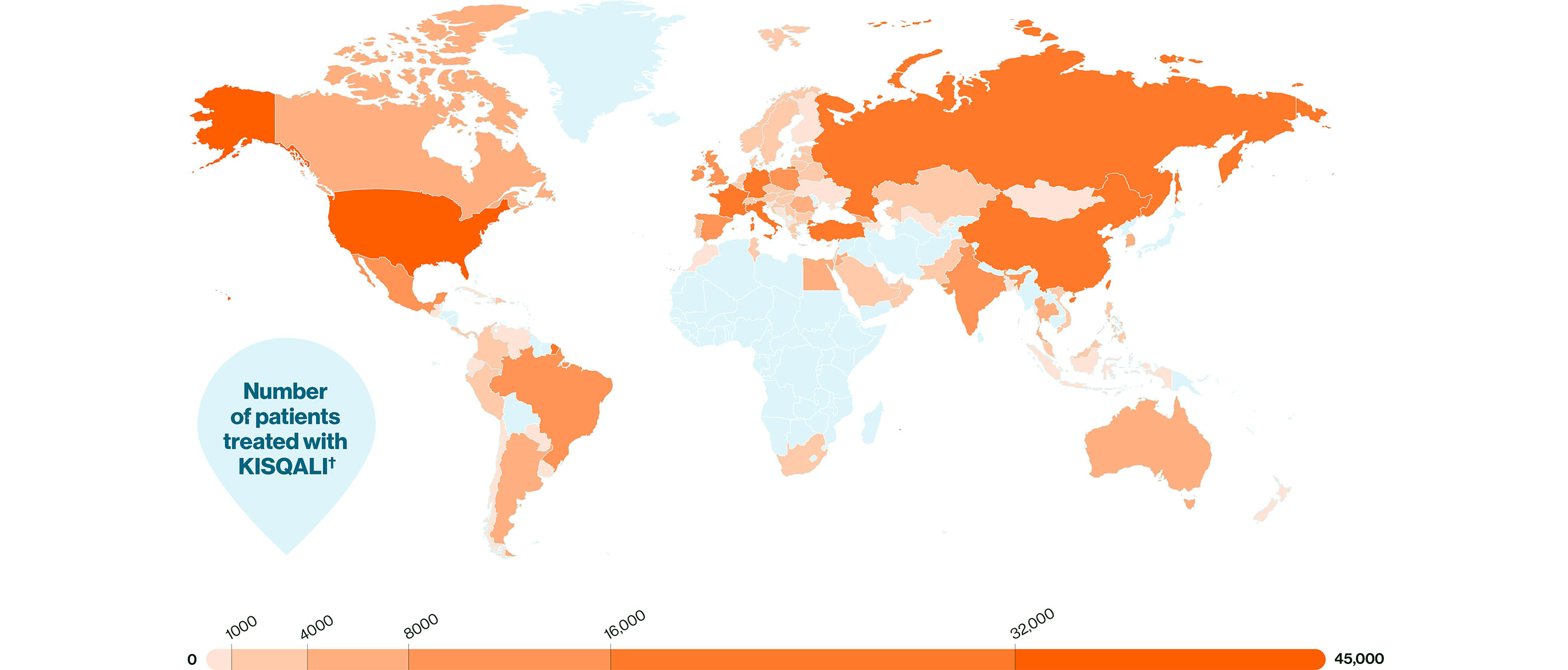
KISQALI is trusted by peers and proven in patients, with over 400,000 having already received KISQALI.*†1,2
Feel assured when prescribing KISQALI: highest-rated CDK4/6i in ABC and highest possible rating in EBC (ESMO-MCBS)‡3
In EBC, ESMO-MCBS scores KISQALI with the highest rating of A for its curative evaluated outcome of DFS.
In ABC, ESMO-MCBS scores KISQALI as the highest-rated CDK4/6i in combination with either an AI or fulvestrant, with a rating of 4 across each phase III trial, due to its consistent and proven OS.


KISQALI + ET lets you keep your patients' well-being at the centre of your treatment strategy, helping them to live life on their terms
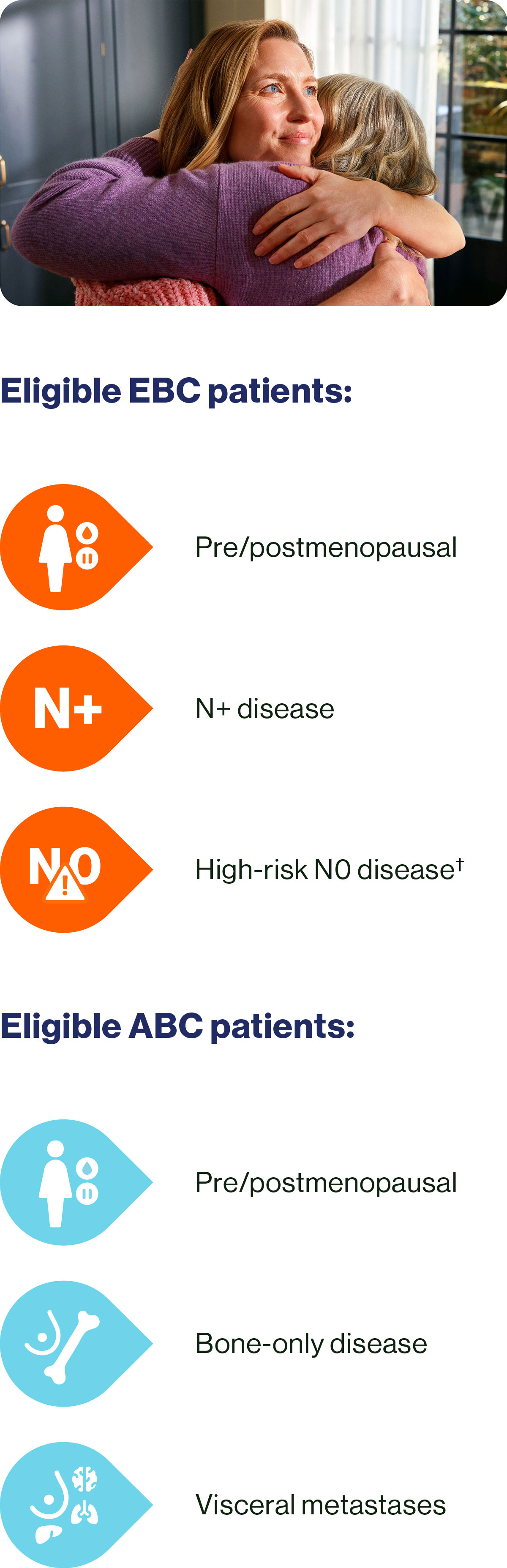
Choose KISQALI + AI to help your eligible EBC patients achieve:
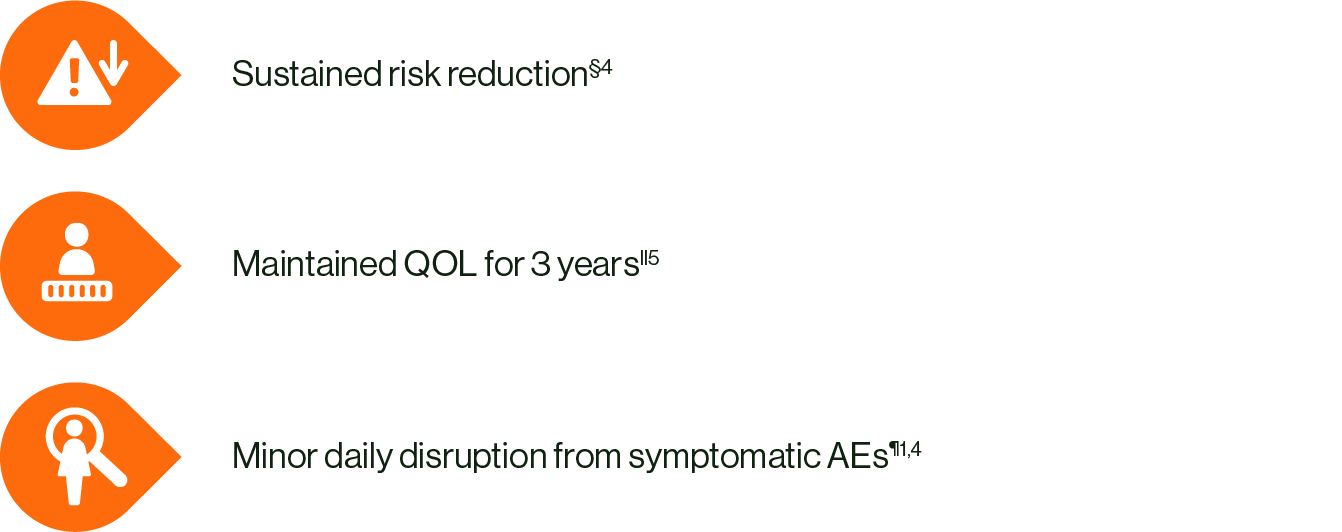
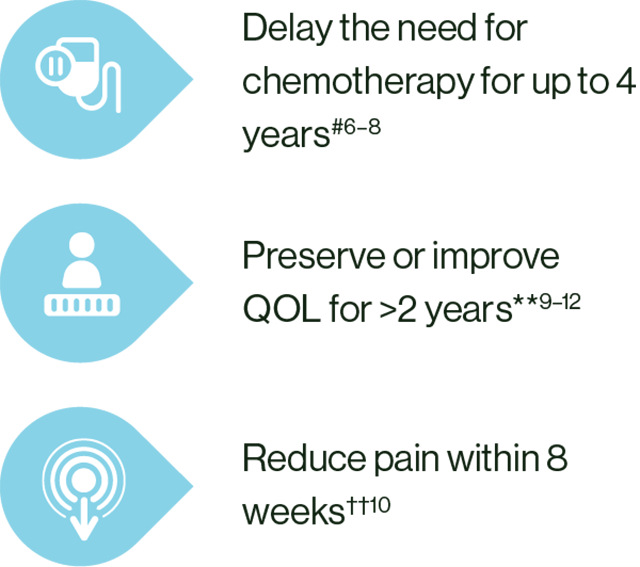
In patients with ABC, KISQALI + ET could help:
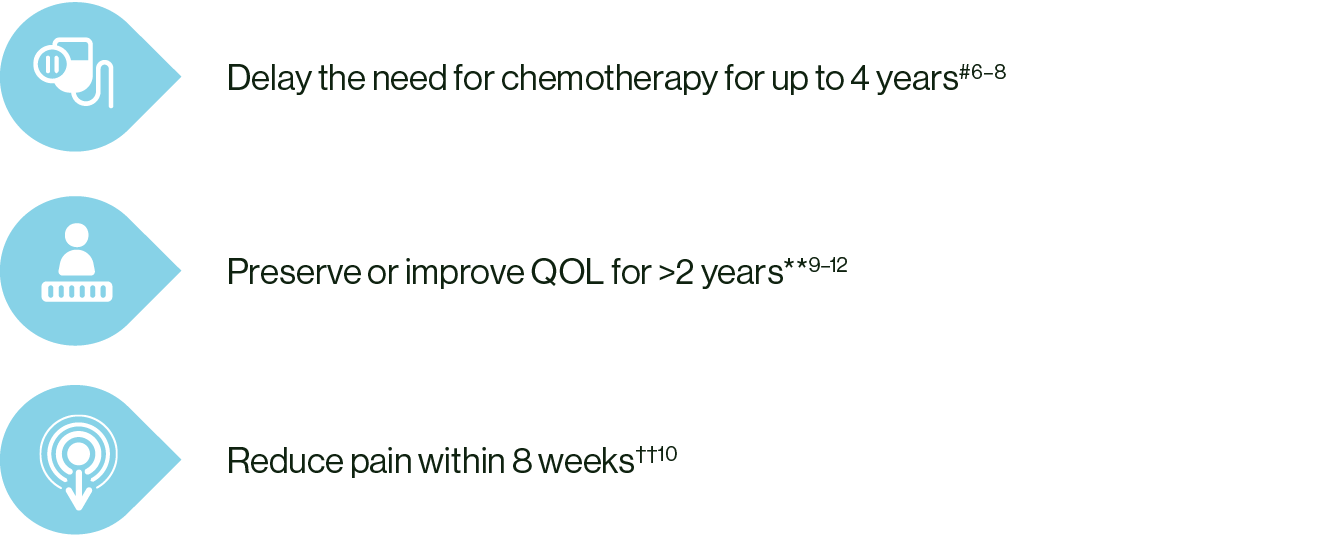
* Proven efficacy (statistically significant improvements in iDFS vs AI in the NATALEE study for EBC and PFS & OS vs ET in the MONALEESA studies) and a manageable safety profile.1
† Worldwide cumulative number of treated patients who have received KISQALI since June 2019.2
‡ In EBC: ESMO-MCBS score of A for KISQALI + AI (NATALEE study). In ABC: Only CDK4/6i with an ESMO-MCBS rating of 4 to achieve OS with both AI and fulvestrant (MONALEESA-2 with KISQALI + AI, MONALEESA-3 with KISQALI + fulvestrant and MONALEESA-7 with KISQALI + ET).3 ABC: score range 1–5 where 5 is highest; 4 and 5 indicate substantial benefit. EBC: score range A–C where A is the highest; A and B indicate substantial benefit.3
§ Data from the NATALEE study. Statistically significant improvement in iDFS with KISQALI + AI vs AI alone (p<0.0001) over 4 years HR 0.715 (95% CI: 0.609–0.840); ARR = 4.9%. 4
ll KISQALI + AI vs AI alone. QOL was assessed in the NATALEE study using the EORTC QLQ-C30.5
¶ The majority of AEs were transient, manageable, and mostly reversible with dose reduction or interruption. The most common AEs across the NATALEE study with a reported frequency ≥20% were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests.1 Please refer to your local SmPC for further information about adverse events and special warnings and precautions for use.
# KISQALI + ET vs ET alone. MONALEESA-2: HR 0.74; 95% CI: 0.61–0.91; MONALEESA-3: HR 0.70; 95% CI: 0.57–0.88; MONALEESA-7: HR 0.69; 95% CI: 0.56–0.87.6–8
** KISQALI + ET vs ET alone. MONALEESA-2: HR 0.94; 95% CI: 0.72–1.24; MONALEESA-3: HR 0.81; 95% CI: 0.62–1.06; MONALEESA-7: HR 0.67; 95% CI: 0.52–0.86.9–12
†† In a post-hoc analysis of MONALEESA-2, a clinically meaningful reduction (>5 point change from baseline) in pain was seen as early as Week 8 and maintained for up to 15 cycles of KISQALI.10
KISQALI in combination with an AI is indicated for the adjuvant treatment of patients with HR+/HER2– EBC at high risk of recurrence. In pre- or perimenopausal women, or in men, the AI should be combined with an LHRH agonist.1 It is also indicated for the treatment of women with HR+/HER2– locally advanced or metastatic breast cancer in combination with an AI or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy. In pre- or perimenopausal women, the ET should be combined with an LHRH agonist.1 KISQALI should not be co-administered with tamoxifen.1


* Consistent results across a broad range of HR+/HER2– eligible patients with statistically significant improvements in iDFS (EBC), PFS and OS (ABC) vs AI in the NATALEE study and ET in the MONALEESA studies.1 KISQALI is not recommended to be used in combination with tamoxifen.1
Explore the data behind the Powerful Consistency* of KISQALI
In HR+/HER2– EBC: You can set the standard in adjuvant care with KISQALI + AI.4
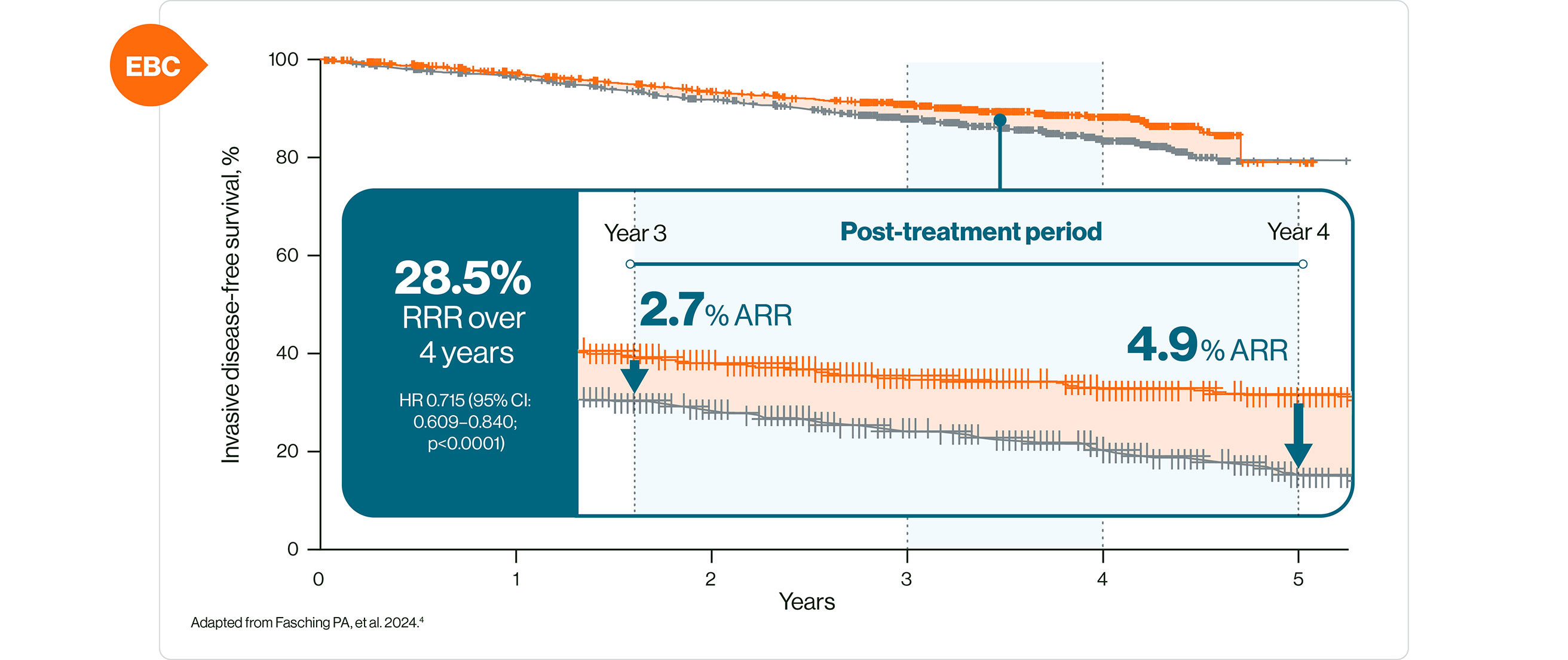
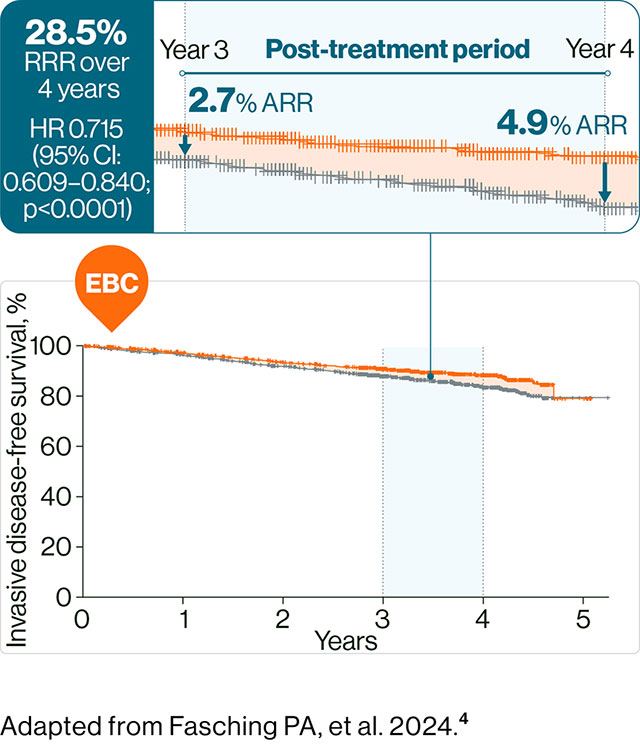
The NATALEE study was a multicentre, randomised, open-label phase III clinical trial of KISQALI + AI vs AI alone in the adjuvant treatment of HR+/HER2– EBC. N=5101. Patients received KISQALI 400 mg/d + AI for 3 years while AI continued ≥5 years. Any (neo)adjuvant ET was permitted for ≤1 year prior to randomisation. Primary endpoint was iDFS.4
In HR+/HER2– ABC: KISQALI is the only CDK4/6i to provide consistent and significant OS across three phase III trials, the gold standard in oncology trials.6,8,13–15
No head-to-head trials exist. This statement is based on evidence from the phase III clinical trials of the MONALEESA trial programme. Trial designs and populations differ across CDK4/6i studies
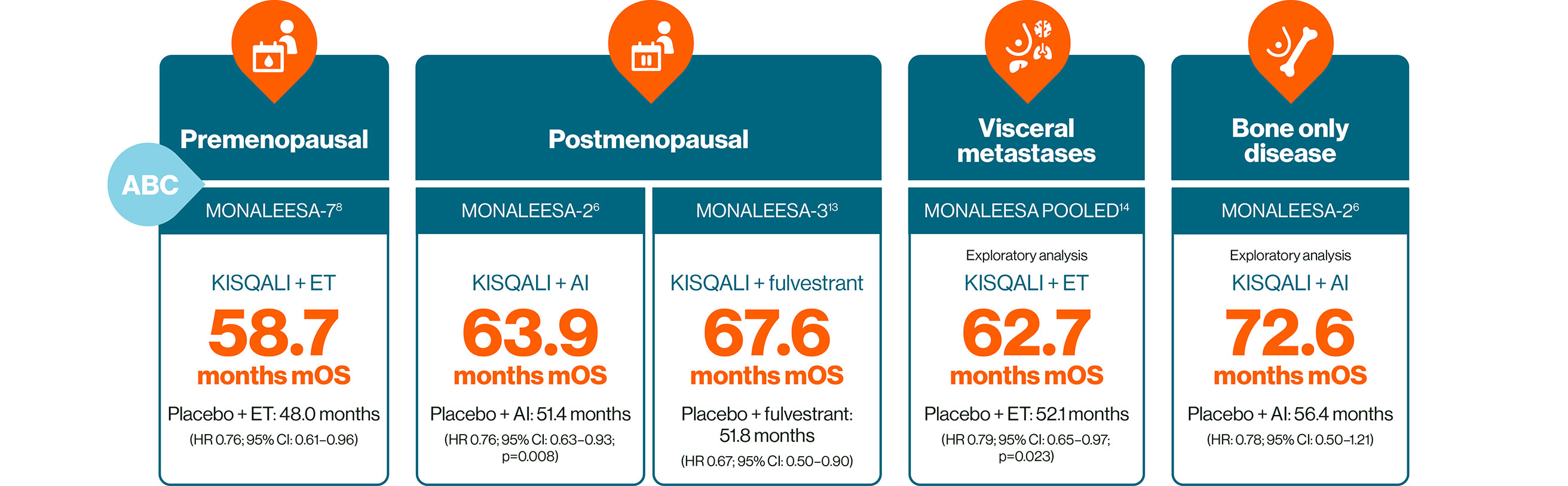
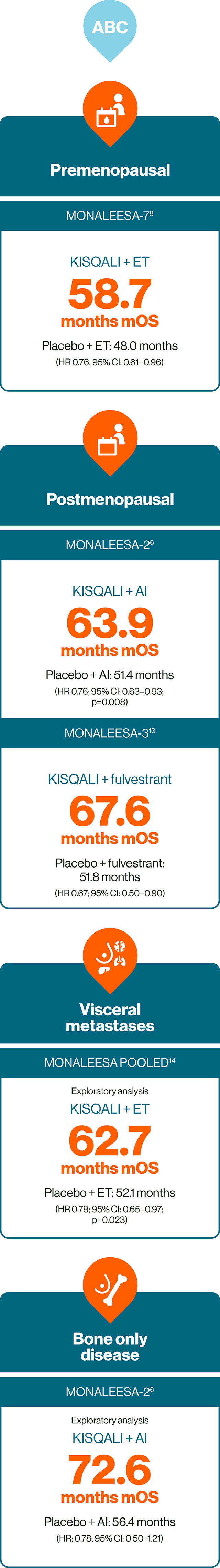
Further studies have also provided evidence for the safety and efficacy of KISQALI + ET in HR+/HER2– ABC, including RIGHT Choice in premenopausal patients, and the real-world studies RIBANNA and CompLEEment.16–18
KISQALI + ET can help you deliver consistent outcomes across both EBC and ABC5,9–12
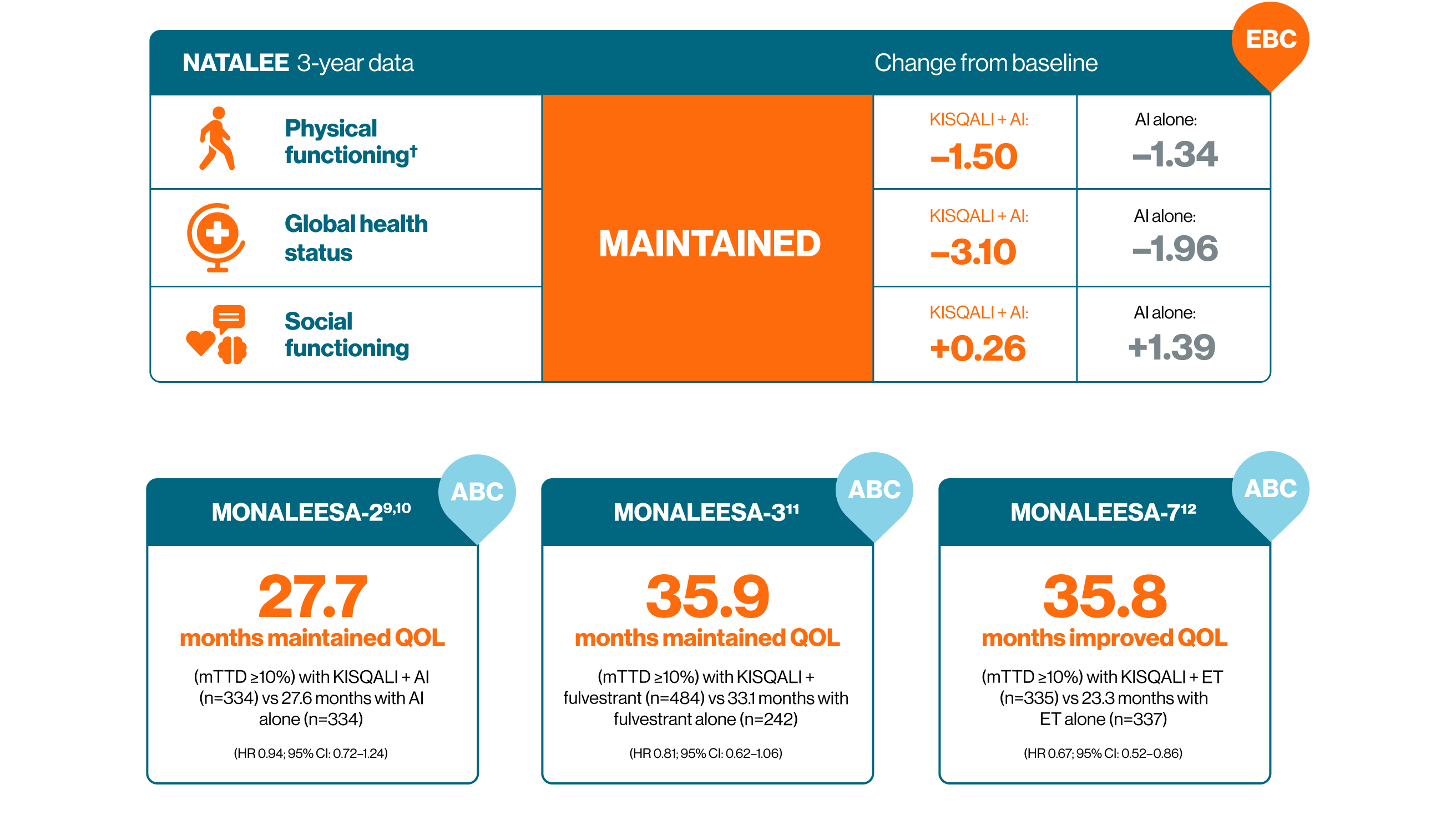
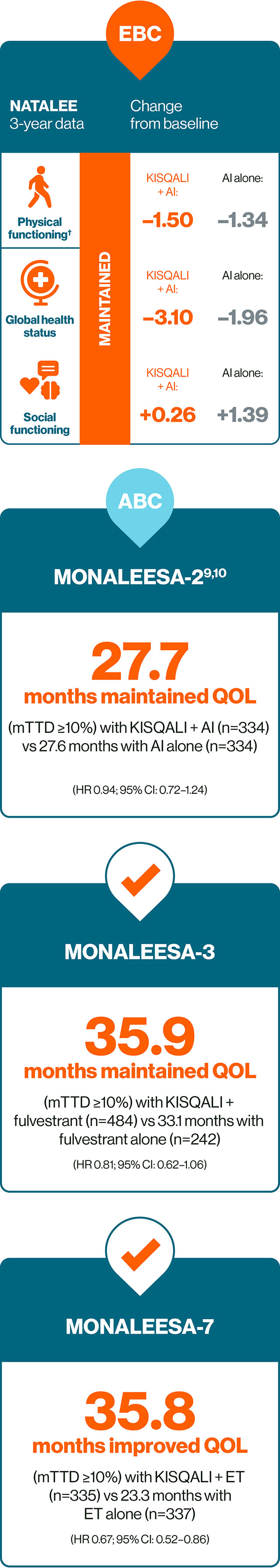
† No difference from baseline was observed in either arm based on established thresholds for interpreting changes in physical functioning score (−5 to 2, no difference).5
KISQALI in combination with an AI is indicated for the adjuvant treatment of patients with HR+/HER2– EBC at high risk of recurrence. In pre- or perimenopausal women, or in men, the AI should be combined with an LHRH agonist.1 It is also indicated for the treatment of women with HR+/HER2– locally advanced or metastatic breast cancer in combination with an AI or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy. In pre- or perimenopausal women, the ET should be combined with an LHRH agonist.1 KISQALI should not be co-administered with tamoxifen.1


* Consistent results across a broad range of HR+/HER2– eligible patients with statistically significant improvements in iDFS (EBC), PFS and OS (ABC) vs AI in the NATALEE study and ET in the MONALEESA studies.1 KISQALI is not recommended to be used in combination with tamoxifen.1
How could KISQALI help you give more of your patients good news?
You can give KISQALI + AI to a wide range of your HR+/HER2– EBC and ABC patients.1

KISQALI can make a meaningful difference to your patients' lives
In EBC: KISQALI has a manageable safety profile with mainly reversible and mostly asymptomatic AEs.§1,4
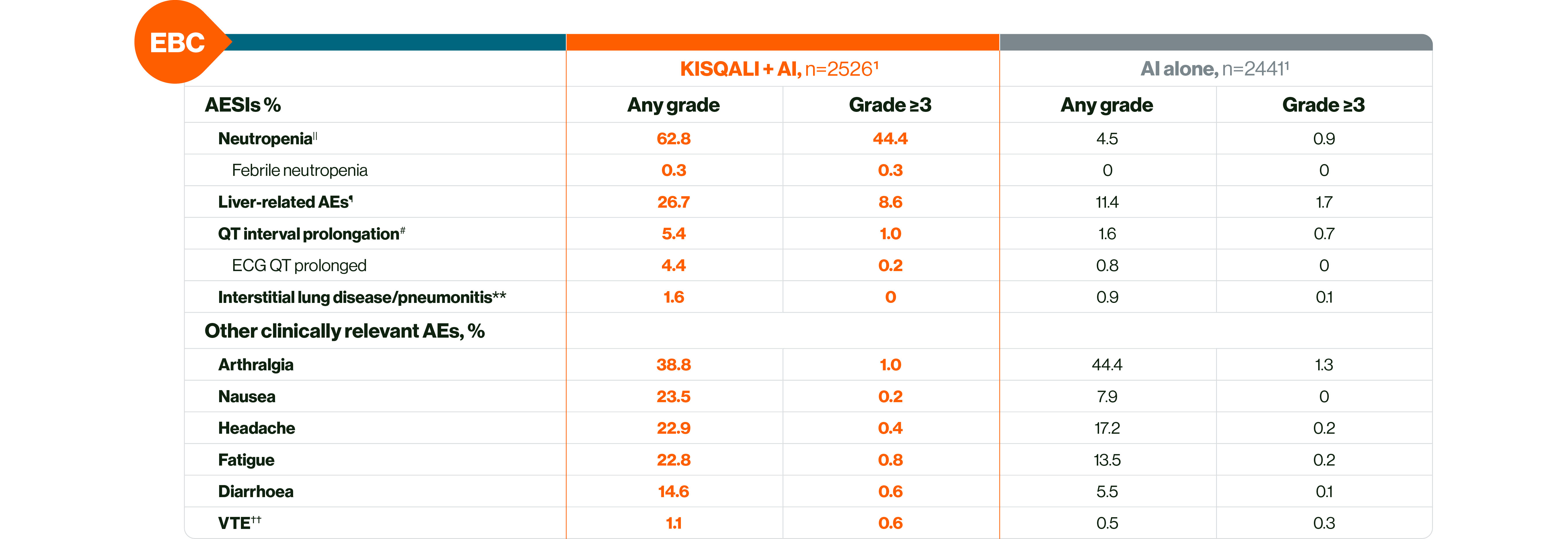
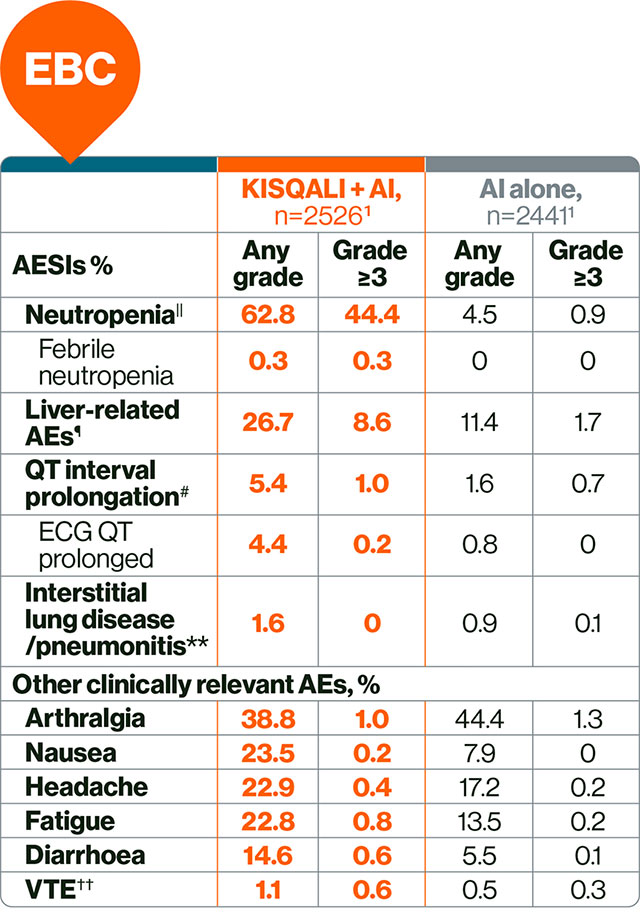
The most common AEs across the NATALEE study with a reported frequency ≥20% were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests.1
In ABC: KISQALI AEs are manageable, mostly asymptomatic, and are reversible with simple dose reduction or interruption.1,19
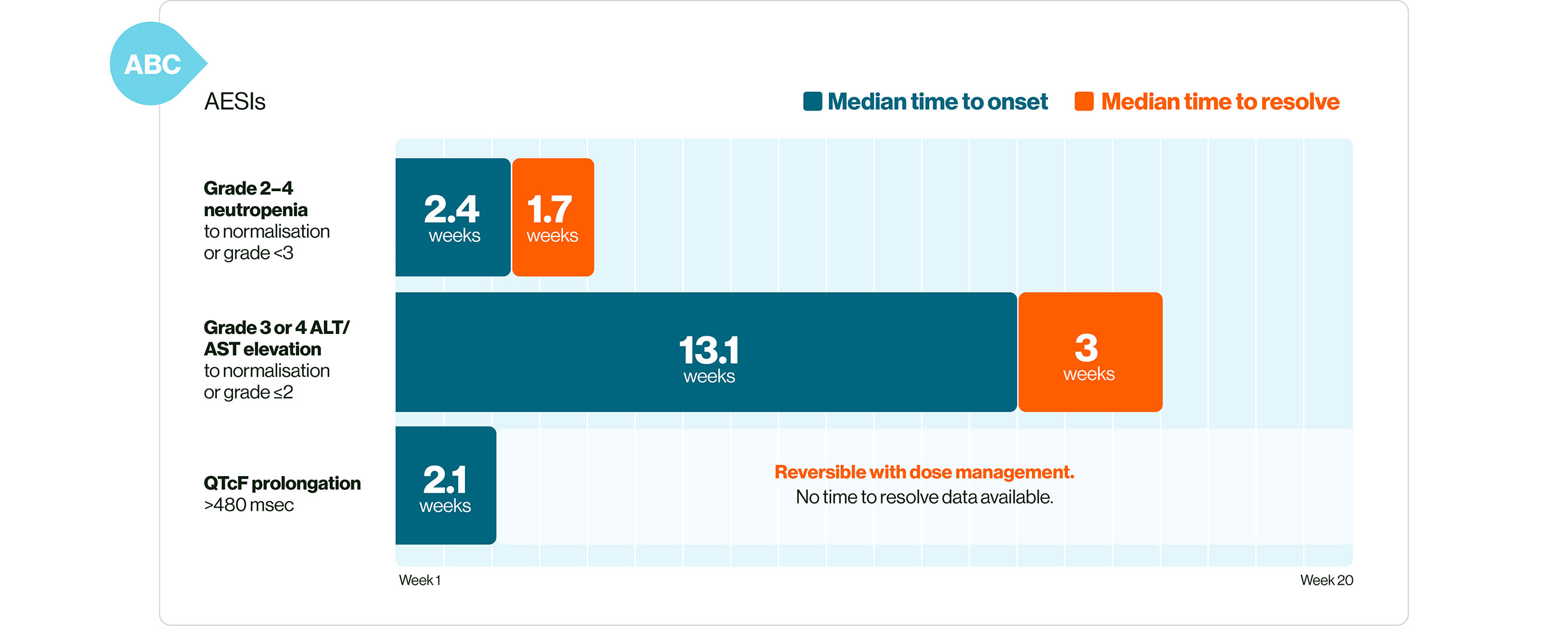
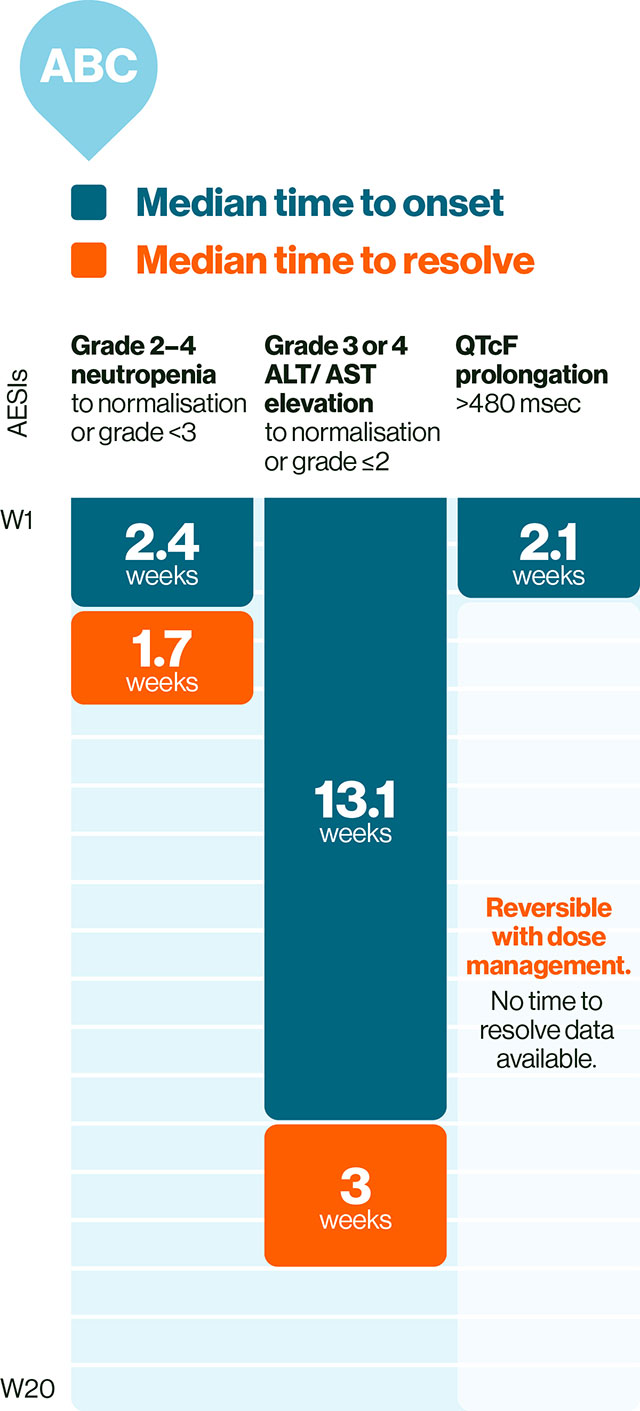
Please refer to the SmPC for guidance about special warnings, precautions for use and management of adverse events.
Ensure your EBC patients aren't left behind
You can offer KISQALI to every eligible patient who began ET within the last year.


Show your patients the benefits of the first 3-year CDK4/6i
Could KISQALI + AI help give your patients longer-lasting reassurance?


† Grade 2 with additional risk factors, such as Ki-67 score ≥20% or defined as an Oncotype DX Breast Recurrence Score of ≥26, or Prosigna/PAM50, MammaPrint, or EndoPredict EPclin high-risk scores, or grade 3.20
‡ vs AI in the NATALEE study and ET in the MONALEESA studies. Consistent results across a broad range of HR+/HER2– eligible patients with statistically significant improvements in iDFS (EBC), PFS and OS (ABC). KISQALI should not be co-administered with tamoxifen.1
§ The majority of AEs were transient, manageable, and mostly reversible with dose reduction or interruption. The most common AEs across the NATALEE study with a reported frequency ≥20% were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests.1
|| This is a grouped term that combines neutropenia and neutrophil count decreased.4
¶ This is a grouped term that includes all preferred terms identified by standardised MedDRA queries for drug-related hepatic disorders.4 Liver-related AEs were predominantly ALT/AST elevations without concomitant bilirubin increase.4
# This is a grouped term.4
•• This is a grouped term that includes all preferred terms identified by standardised MedDRA queries for interstitial lung disease.4
†† Grouped term that includes all preferred terms identified by standardised MedDRA queries for venous thromboembolism.4
‡‡ Data from the NATALEE trial. Statistically significant improvement in iDFS with KISQALI + AI vs AI alone (p<0.0001) over 4 years.4
KISQALI in combination with an AI is indicated for the adjuvant treatment of patients with HR+/HER2– EBC at high risk of recurrence. In pre- or perimenopausal women, or in men, the AI should be combined with an LHRH agonist.1 It is also indicated for the treatment of women with HR+/HER2– locally advanced or metastatic breast cancer in combination with an AI or fulvestrant as initial endocrine-based therapy, or in women who have received prior endocrine therapy. In pre- or perimenopausal women, the ET should be combined with an LHRH agonist.1
KISQALI should not be co-administered with tamoxifen.1
Discover more about the Powerful Consistency of KISQALI here
The most common AEs across the NATALEE study with a reported frequency ≥20% were neutropenia, infections, nausea, headache, fatigue, leukopenia and abnormal liver function tests.1 The most common AEs across the pooled MONALEESA studies with a reported frequency ≥20% were neutropenia, infections, nausea, fatigue, diarrhoea, leukopenia, vomiting, headache, constipation, alopecia, cough, rash, back pain, anaemia and abnormal liver function tests.1
MONALEESA-2: N=668, double-blind, placebo-controlled, 1:1 randomised, multicentre, phase III trial in postmenopausal women for 1L treatment of HR+/HER2– ABC. KISQALI 600 mg/d or placebo orally (3 weeks on/1 week off) + AI (letrozole 2.5 mg continuous). Primary endpoint was PFS, key secondary endpoint was OS.6
MONALEESA-3: N=726, double-blind, placebo-controlled, 2:1 randomised, phase III trial in postmenopausal women for 1L or 2L treatment of HR+/HER2– ABC plus those with early relapse. KISQALI 600 mg/d or placebo orally (3 weeks on/1 week off) + 500 mg intramuscular fulvestrant. Primary endpoint was PFS, key secondary endpoint was OS.13
MONALEESA-7: N=672, double-blind, placebo-controlled, 1:1 randomised, phase III trial in pre- or perimenopausal women for 1L treatment of HR+/HER2– ABC in patients who received ≤1 lines of chemotherapy for ABC. KISQALI 600 mg/d or placebo orally (3 weeks on/1 week off) + AI (letrozole 2.5 mg or anastrozole 1 mg) or tamoxifen 20 mg orally once daily continuously + LHRH agonist (goserelin 3.6 mg subcutaneously on Day 1 of every cycle).* Primary endpoint was PFS, key secondary endpoint was OS.8
MONALEESA pooled analysis included 1124 patients with visceral metastases (including liver metastases or ≥3 disease sites) from across the MONALEESA trials, 714 of these patients received 1L treatment and are included here (395 patients received KISQALI + ET). These data are exploratory and hypothesis-generating only.14
NATALEE: N=5101, multicentre, randomised, open-label phase III clinical trial of KISQALI + AI vs AI alone in the adjuvant treatment of HR+/HER2– EBC. KISQALI 400 mg/d + AI for 3 years while AI was continued for ≥5 years. Any (neo)adjuvant ET was permitted for ≤1 year prior to randomisation. Men and premenopausal women also received goserelin. Primary endpoint was iDFS, key secondary endpoints were DDFS and OS.4
* KISQALI should not be co-administered with tamoxifen.1
1L, first-line; 2L, second-line; ABC, advanced breast cancer; AE, adverse event; AESI, adverse event of special interest; AI, aromatase inhibitor; ALT, alanine aminotransferase; ARR, absolute risk reduction; AST, aspartate aminotransferase; CDK4/6i, cyclin-dependent kinase 4 and 6 inhibitor; CI, confidence interval; DDFS, distant disease-free survival; DFS, disease-free survival; EBC, early breast cancer; ECG, electrocardiogram; ESMO, European Society for Medical Oncology; ET, endocrine therapy; HER2–, human epidermal growth factor receptor 2 negative; HR+, hormone receptor positive; iDFS, invasive disease-free survival; LHRH, luteinising hormone-releasing hormone; MCBS, Magnitude of Clinical Benefit Scale; mOS, median overall survival; N+, node positive; N0, node negative; OS, overall survival; PFS, progression-free survival; QOL, quality of life; RRR, relative risk reduction; VTE, venous thromboembolism.
References
1. KISQALI (ribociclib). Summary of Product Characteristics.
2. Novartis internal sales data. 2024.
3. ESMO. ESMO-MCBS scorecards. Available at: https://www.esmo.org/guidelines/esmo-mcbs/esmo-mcbs-for-solid-tumours/esmo-mcbs-scorecards?
mcbs_score_cards_form%5BsearchText%5D=&mcbs_score_cards_form%5Btumour-type%5D=0. Accessed November 2025.
4. Fasching PA, et al. Oral LBA13. Presented at the European Society for Medical Oncology Congress 2024. 13–17 September, Barcelona, Spain.
5. Fasching PA, et al. Clin Cancer Res. 2025;31(9):1625–1635.
6. Hortobagyi GN, et al. N Engl J Med. 2022;386(10):942–950.
7. Slamon DJ, et al. Ann Oncol. 2021;32(8):1015–1024.
8. Lu Y-S, et al. Clin Cancer Res. 2022;28:851–859.
9. Beck JT, et al. Cancer Res. 2019;79 (4_Supplement):P6-18-14.
10. Verma S, et al. Breast Can Res Treat 2018;170:535–545.
11. Fasching PA, et al. Breast. 2020;54:148–154.
12. Harbeck N, et al. Ther Adv Med Oncol. 2020;12:1–8.
13. Neven P, et al. Breast Cancer Res. 2023;25:103.
14. Yardley DA, et al. Ann Oncol. 2022;33(S7):S629.
15. Merino M, et al. J Clin Oncol. 2023;41(5):2706–2713.
16. Lu Y-S, et al. J Clin Oncol. 2024;42(23):2812–2821.
17. Decker T, et al. ESMO Open. 2025;10(6):105105.
18. De Laurentiis M, et al. Breast Cancer Res Treat. 2021;189(3):689–699.
19. Burris HA, et al. Br J Cancer. 2021;125:679–686.
20. Slamon DJ, et al. Ther Adv Med Oncol. 2023;15:1–16.
Click here for Prescribing Information.
Click here for adverse event reporting in your country.
Novartis AG
This site is intended for Healthcare Professionals only.
Last updated November 2025.
©2025 Novartis
All rights reserved.
FA-11502761-1 | November 2025